Australians can now access a combination immunotherapy drug with better survival rates than previously covered treatments.
A dual immunotherapy (Opdualag, Bristol-Myers Squibb) for unresectable or metastic melanoma will be available to patients on the PBS from 1 February.
Federal Health Minister Mark Butler made the announcement at a press conference held at The Queen Elizabeth Hospital, Adelaide, last weekend.
“While great progress has been made in the treatment of advanced melanoma, we need more treatment options that are affordable,” said Ms Tamara Dawson, founder of the Melanoma & Skin Cancer Advocacy Network (MSCAN).
“Australians living with advanced melanoma and their families therefore welcome the PBS listing of a new combination immunotherapy treatment on the PBS.”
The fixed dose nivolumab/relatlimab combination (160 mg / 480 mg IV every four weeks) is for patients aged 12 and over, weighing at least 40kg, with unresectable Stage III or IV malignant melanoma, not previously treated with a PD-1 inhibitor for this indication. If treated for resected Stage IIIB, IIIC, IIID, or IV melanoma with an adjuvant PD-1 inhibitor, they must not have experienced disease progression while on the treatment or had disease recurrence within six months of completion of the treatment.
Nivolumab is a programmed cell death protein 1 (PD-1) inhibitor, and relatlimab is a novel first-in-class lymphocyte-activation gene 3 (LAG-3)-blocking antibody.

The manufacturer says LAG-3 and PD-1 are often present in immune cells in people with metastatic melanoma, contributing to T-cell exhaustion. The drug supports T-cell function by inhibiting the two immune checkpoints.
“Over the past decade, the use of drugs that inhibit immune checkpoints has changed how unresectable or metastatic melanoma is treated, making long-term survival a real possibility for patients. … [This] approval is particularly significant, as it shows commitment and focus on bringing innovative drug therapy options to patients, and targeting two different immune checkpoints – LAG-3 and PD-1 – does just that,” said Professor Georgina Long AO, co-medical director of Melanoma Institute Australia.
Alternative drugs on the PBS are nivolumab monotherapy and pembrolizumab (also a PD-1 inhibitor) monotherapy. Trials show that the nivolumab/relatlimab combination leads to better median progression-free survival than nivolumab alone (10.1 months vs 4.6 months) and at 12 months (47.7% compared with 36.0%).
A combination nivolumab/ipilimumab therapy is approved by the TGA, but not on the PBS. There are no direct comparisons between the two combination therapies. The Pharmaceutical Benefits Advisory Committee (PBAC) found the relatimib/nivolumab combination to be non-inferior in terms of efficacy and safety compared with the nivolumab/ipilimumab combination.
Melanoma treatment guidelines, both Australian and international, recommend combination therapy over PD-1 inhibitor monotherapy for unresectable stage III or IV metastatic melanoma.
From 1 February 2024, metastatic melanoma treatments available in Australia are:
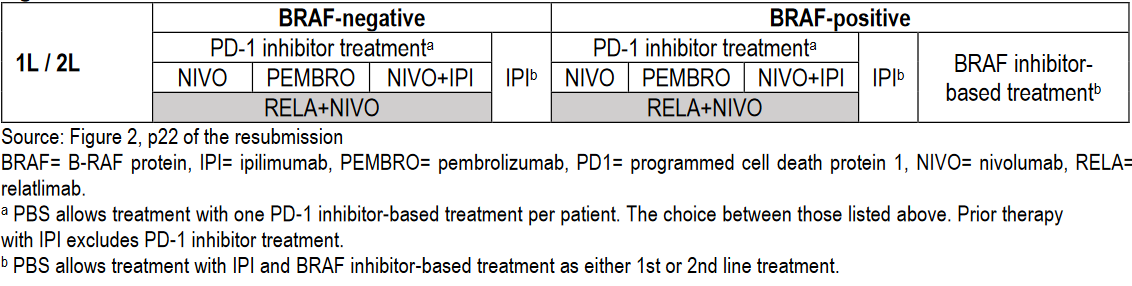
(Source: Public Summary Document – March 2023 PBAC Meeting with July 2023 Addendum)
The relatimib/nivolumab treatment is associated with greater incidence and severity of immune-related adverse reactions compared with nivolumab monotherapy, with 22% experiencing grade 3/4 treatment-related adverse events, compared with 12% of patients on nivolumab alone. In trials, 14% of patients treated with nivolumab/relatlimab discontinued treatment due to adverse events, and 6% of patients receiving nivolumab.
The PBAC summary noted that it recommended patients be monitored at least before each dose. It also cautions that some patients can have a transient tumour flare and disease response in the first few months after starting immunotherapy and, where suspected, these patients should be scanned from four weeks post commencement.