The genomic testing guidelines are ‘sensible’ and reflect Australian practice, according to one expert.
The American Society of Clinical Oncology released new guideless for genomic testing in metastatic prostate cancer earlier this year, which will serve as a reminder for clinicians to undertake genomic testing in these patients.
Tumour genetics have traditionally not been a key focus in the treatment of prostate cancer, which in a stark contrast to other types of cancer such as breast and lung. As a result, treatment approaches may not be guided by precision medicine.
To address this shortcoming, the American Society of Clinical Oncology have developed a new guideline around germline and somatic genomic testing for metastatic prostate cancer. The guideline was published in the Journal of Clinical Oncology earlier this year.
Professor Arun Azad, a medical oncologist and translational researcher at the Peter MacCallum Cancer Centre, said the guidelines aligned with Australia’s approach to germline and somatic testing for patients with metastatic prostate cancer.
“[But] it will help to reinforce the importance of doing this testing, both in terms of assessing patient suitability for treatment with PARP inhibitors, but also to identify any germline mutations in BRCA or similar genes that could have implications for family members of the patient with prostate cancer,” Professor Azad told Oncology Republic.
A panel of international and multidisciplinary experts and patient representatives selected 14 papers – eight systematic reviews and six clinical trials – published between 2020 and 2024 to serve as the evidence base for the new guidelines, which addressed six overarching clinical questions.
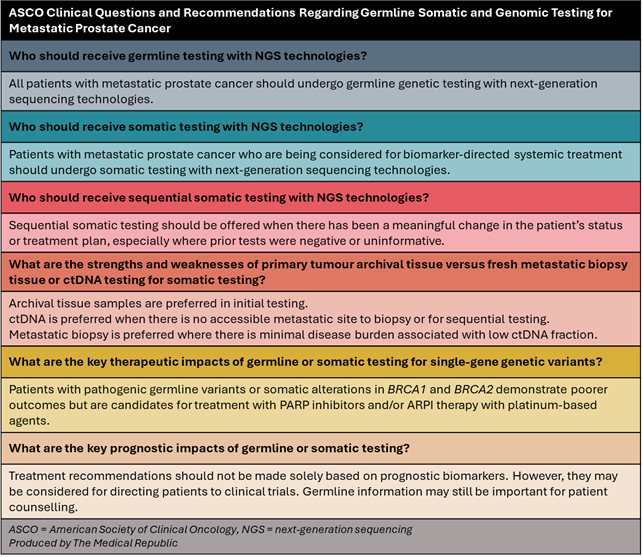
Four of the six recommendations were classified as “strong” based on the high-quality evidence available in the literature. There was low- to moderate-quality evidence for the questions regarding who should receive sequential somatic testing and the strengths and weaknesses of archival/fresh tissue versus ctDNA testing, meaning the panel were less confident in their recommendations for these items.
Professor Azad said the guidelines were sensible and supported clinical practice, but highlighted a key difference in the use of ctDNA testing between the two nations.
“In the United States increasingly ctDNA is being used for repeat genetic testing in patients with prostate cancer, as well as fact other cancers. We would like to do this in Australia, but unfortunately there is no approved circulating tumour DNA test, and no test is reimbursed either. Hopefully this is something that will change in the future,” he said.
The panel noted that although much of the data used to inform the new guideline came from randomised phase 3 trials, this meant the recommendations may not represent the more diverse cohort of patients seen in clinical practice.
“Moreover, these data lack the reporting granularity of aspects such as somatic versus germline, zygosity [and] functionality of alterations,” the panel noted.
Professor Azad said that while Australia tended to follow international guidelines on genomic testing for prostate cancer, funding for such testing was available for certain patients.
“There is funding for testing patients with metastatic castration-resistant prostate cancer who have progressive disease on an androgen receptor pathway inhibitor and are being considered for therapy with olaparib, a PARP inhibitor,” he told OR.
“The other mechanism for doing genetic testing for prostate cancer is through the Molecular Screening and Therapeutics (MoST) program run by Omico, which is available to all patients with advanced cancer across Australia.”
The Australian Institute for Health and Welfare’s Cancer data in Australia report estimates over 26,000 men were diagnosed with prostate cancer last year, making it the most commonly diagnosed cancer for males and for Australia overall. The number of new prostate cancer cases is estimated to surpass 31,000 in 2034.
Data from the AIHW indicates that while prostate cancer mortality rates have declined over the past three decades (from 62 deaths per 100,000 males in 1994 to 33 per 100,000 in 2024), the number of deaths over the same period of time has risen from approximately 2700 to 3900.


